Diethyl allyl phosphate, allyl diethyl phosphate (DEAP)
Product Manager:Nick Wilde

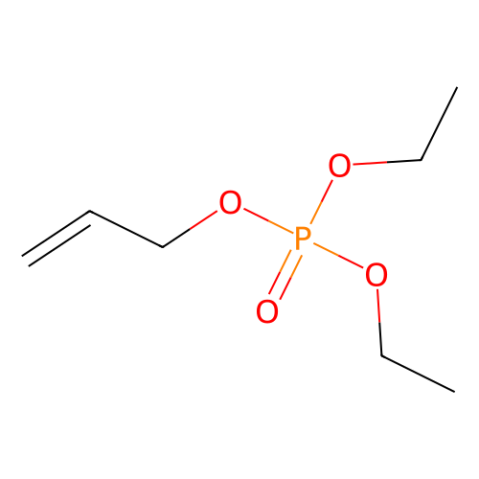
Diethyl allyl phosphate (DEAP) serves as a hydrogen acceptor and can be utilized in Pd-catalyzed oxidations, among other applications.
Recent Literature
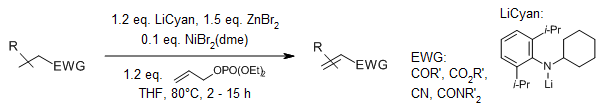
Nickel(II) bromide, dimethoxyethane adduct catalysis facilitates the α,β-dehydrogenation of carbonyl compounds. Furthermore, an oxidative cycloalkenylation reaction allows for the synthesis of bicycloalkenones featuring fused, bridged, and spirocyclic ring systems, utilizing unactivated ketone and alkene starting materials.
D.Huang, S. M. Szewczyk, P. Zhang, T. R. Newhouse, J. Am. Chem. Soc., 2019, 141, 5669-5674.
https://doi.org/10.1021/jacs.9b02552
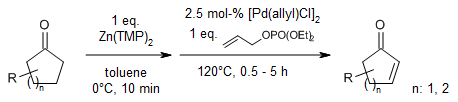
Allyl-palladium catalysis allows for a one-step α,β-dehydrogenation of ketones through their zinc enolates. The refined method employs readily available Zn(TMP)2 as a base and diethyl allyl phosphate as an oxidant, operates without the need for added salts, and is compatible with a wide range of cycloalkanones.
D.Huang, Y. Zhao, T. R. Newhouse, Org. Lett., 2018, 20, 684-687.
https://doi.org/10.1021/acs.orglett.7b03818
Quoted from: https://www.organic-chemistry.org/chemicals/oxidations/diethyl-allyl-phosphate-deap.shtm
Aladdin:https://www.aladdinsci.com
 首页
首页 400-620-6333
400-620-6333
